Risk Management
| << Back | Download |
Medicolegal Implications of Using Off-Label Drugs and Devices
By E. Randy Craven, MD, and Elizabeth C. Moran, JD
Digest, Winter, 1996
Most physicians have heard these words: “Doctor, isn’t there anything else you can do? Some new treatment you can try?” Patients often perceive “new” treatments as better or less problematic than existing treatments and pleas for something “new” are particularly understandable when patients are faced with a terminal condition or loss of vision. Physicians generally look for proven treatments that will give their patients the best results with the least side effects. However, because of the tremendous psychological leverage of offering something “new,” physicians might be tempted to try unproven treatments to keep up with market forces. A good example of how market forces lead to such decisions might go something like this: “Mrs. Jones, you have the privilege of being in the right place at the right time. I am excited to inform you that I use the latest technology. The old method of correcting myopia is out and my new technique is in. In fact, because of my commitment to stay on the cutting edge, the laser I will be using to reshape your cornea was designed by me with the help of an engineer. This laser will correct your myopia in minutes.”
What assurance does Mrs. Jones have that the laser developed by her ophthalmologist is safe? Is it prudent for physicians to develop their own equipment or drugs for use on their patients? Who or what regulates physicians who do so?
Regulation of Physicians’ Practices
Production, sale, and clinical research of new drugs and medical devices are subject to regulation by the Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS). Physicians and patients are protected to some degree when they use drugs or devices that have undergone the scrutiny of the FDA and received approval for marketing and sale. The known indications, hazards, and adverse effects of the approved device or drug are required to be included in the product labeling. The FDA can restrict the ability of a company to sell, manufacture, or import a drug or device and can impose a variety of other protective measures.
Ordinarily, physicians are not directly regulated in their use of drugs and devices in day-to-day practice but are expected to practice in a manner that is designed solely to insure the well-being of the patient. Unless a physician is himself marketing or selling a drug or device, or acting as an investigator in clinical research, the FDA generally does not oversee or interfere with a physician’s individual practice decisions.
It is a different matter when the physician is conducting clinical research. Research activity occurs when the clinician conducts a “systematic investigation” designed to develop or contribute to “generalizable knowledge” such as by testing a hypothesis, drawing conclusions, and developing a base of knowledge from the results, especially concerning safety or effectiveness of the product.
If a practicing physician departs from usual (standard) treatments for an individual patient, such as by using a self-made device, a modified device, or a marketed drug for an off-label use, such use does not in and of itself constitute “research.” However, the non-standard treatment might constitute a deviation from accepted standards of medical care. The standard of medical care, which is based upon what reasonable physicians in the same specialty would do at the same time under similar circumstances, is overseen by state and local medical boards, and indirectly, by malpractice lawsuits. Issues of deviation from standards of care are usually raised by patients and/or other practitioners filing a complaint with their state or local medical board or by patients bringing malpractice lawsuits. The medical board also may be informed of aberrant practice styles by federal agencies, such as the Health Care Financing Administration and Medicare Peer Review Organizations.
In addition, local hospitals and clinics oversee standards of medical practice through credentialing, peer review, and quality assurance procedures, which involve investigations of standards of care, identification of deficiencies, and establishment of minimum qualifications for privileges. This is why malpractice insurance companies request information relating to licensing sanctions, peer review proceedings, and denial or surrender of hospital privileges.
So Mrs. Jones is protected by community standards, state and local agencies and institutions, and to a lesser degree, by agencies of the federal government.
Physician Practice versus Investigational Use
A physician may manufacture his or her own equipment or devices solely for his own use in his practice, and may use approved products for non-approved (off-label) applications. In fact, off-label use of medication is quite common. Distinguishing off-label use of drugs or devices as part of a physician’s practice from “experimental” or “investigational” use may be difficult at times. If the new use is based on firm scientific rationale and sound medical evidence, and is not for the purpose of developing information about safety or efficacy (for example, to support a request for FDA approval of the new use or to support advertising of new uses for the product), the use generally will qualify as “the practice of medicine” rather than “investigational use.”
However, if the physician is gathering new information on multiple patients, particularly for publication purposes or to obtain approval for a new device or new use, it is probably considered research, and the physician must comply with the panoply of federal statutes and regulations governing all aspects of approval of new drugs and devices, including numerous requirements for the protection of human subjects in research. In most cases, the practitioner will be required to obtain approval from his or her local Institutional Review Board (IRB), a committee that operates under HHS and FDA regulations for the purpose of protecting the rights of research subjects. The FDA will not accept research data in support of a new drug or device application unless the research protocol and consent documents have been reviewed and approved by an IRB. Similarly, peer reviewed journals usually require documentation of IRB approval before research results will be accepted for publication.
The FDA does have the right to request tracking of marketed products and, although off-label uses in medical practice are not generally regulated, the FDA can disapprove an existing product, require additional warnings, and/or request a recall of a product if it is not happy with off-label uses of the product. Otherwise, regulation of a physician’s medical practice generally falls under the jurisdiction of the state and local agencies previously discussed.
Liability and Insurance Issues
If a physician develops a new device, such as a new ultrasound for phacoemulsification to use on patients in the office, he or she may well face additional liability exposure for problems resulting from the use of the device. Injuries caused by the use of non-approved devices and drugs generally fall within the scope of malpractice and general liability coverage, but a physician may be exposed to personal risk as well if the insurance policy does not cover the liability associated with such uses.
For this reason, it is a good idea to contact your professional liability carrier about potential liability exposure if any of your practice activities involve the use of a self-made instrument or off-label use of drugs or devices regardless of whether they have gone through IRB approval. OMIC, for example, has carefully considered the use of the excimer laser in photorefractive keratectomy (PRK) and laser assisted in-situ keratomileusis (LASIK) and has developed internal guidelines intended to help reduce the increased professional liability and exposure to claims associated with their use.
Off-label applications of drugs also increase the potential for liability, although many drugs are widely used this way. A good example is mitomycin-C (Mutamycin) for glaucoma surgery. Mitomycin-C is FDA approved “for the use of disseminated adenocarcinoma in conjunction with other approved chemotherapeutic agents.” There is no mention of using this drug for pterygia or glaucoma surgery, and there is a long list of side effects for this drug, including pulmonary toxicity that does not appear to be dose related. Nevertheless, glaucoma surgeons are using this medication with increasing frequency, and even a few general ophthalmologists are using it to prevent the recurrence of pterygia following excision.
OMIC has considered the use of mitomycin-C and other off-label drugs (such as cyclosporin drops) and has this general recommendation:
When using a new or old drug in an approved manner, proceed with a green light. If it is an existing drug used in a non-approved manner (off-label), first consider whether its use poses significantly increased risks to the patient. Second, consider whether its use can be expected to bring good results without a higher complication rate. If it presents no more risk to the patient than that of daily living, proceed with its use; for example, the use of aspirin for anticoagulation after a central retinal vein occlusion. If there is an increased risk to the patient, ask yourself if at least a reasonable number of physicians in your specialty are using the treatment; that is, have peer reviewed articles been published supporting the use of the new treatment and is the treatment being used by a reasonable number of other practitioners with the same level of training as you?
Ophthalmologists are considered medical “specialists” and in most cases are held to a “national” rather than “local” standard of care. The same is true of subspecialists in various areas of ophthalmology. An ophthalmologist may face increased liability for off-label uses if a reasonable number of similar specialists or subspecialists are not using the same new treatment. An ophthalmologist whose patients experience more problems with off-label use of a medication than they would with standard methods of treatment might well be subject to professional criticism, and thus be exposed to malpractice liability and potential licensure or credentialing actions.
Additional Precautions
When considering new techniques, new devices, or new uses for approved drugs, practitioners need to think about taking additional measures to protect themselves as well as their patients. If you select a treatment for an individual patient with the intent that it will enhance the patient’s well-being, and there is sound medical evidence supporting the treatment, you are likely to be on solid ground. In such cases, FDA regulation is generally not controlling, and you probably will not need IRB approval unless the treatment so departs from standard practices that it may be considered experimental or investigational, or it presents significantly increased risks for the patient, and/or you wish to use the information for study purposes.
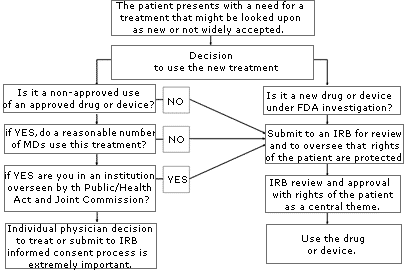
If you are uncertain about whether your use of a treatment or device might be considered “investigational” or “experimental,” consult your local IRB. An IRB is usually available at your local institution (some regional IRBs also exist) to review your proposed use and determine if it constitutes “research” requiring IRB approval. Similarly, if you are unsure whether the treatment has sufficient peer support or is simply too new, or if the literature is unclear, consider going through an IRB to help you with your decision. The flow diagram set forth on page A-78 may help you through the process of determining if you should submit your treatment proposal for IRB review.
Anytime you are considering unapproved uses of a device or drug, the informed consent process and its documentation, including treatment alternatives, should be thorough and specific in case you are later called upon to defend your decision to use non-standard treatments. If you have a specific area of concern, contact OMIC’s Risk Management Department for further information or referral to the appropriate resource or agency.
Please refer to OMIC's Copyright and Disclaimer regarding the contents on this website